Balancing equations gizmo answer key pdf provides a comprehensive guide to understanding and mastering the art of balancing chemical equations. This essential tool empowers students and educators alike to navigate the intricacies of stoichiometry and unravel the mysteries of chemical reactions.
Through engaging simulations and step-by-step instructions, the Balancing Equations Gizmo unlocks the secrets of chemical equilibrium, enabling learners to confidently predict reaction outcomes and delve deeper into the fascinating world of chemistry.
Delving into the Balancing Equations Gizmo simulation, users embark on an interactive journey where they witness the dynamic balancing of chemical equations firsthand. The Gizmo’s intuitive interface and customizable parameters allow learners to experiment with various reactants and products, fostering a deeper comprehension of stoichiometric ratios and the conservation of mass.
By manipulating coefficients and observing the resulting changes, students develop a profound understanding of the fundamental principles governing chemical reactions.
Balancing Equations: Balancing Equations Gizmo Answer Key Pdf
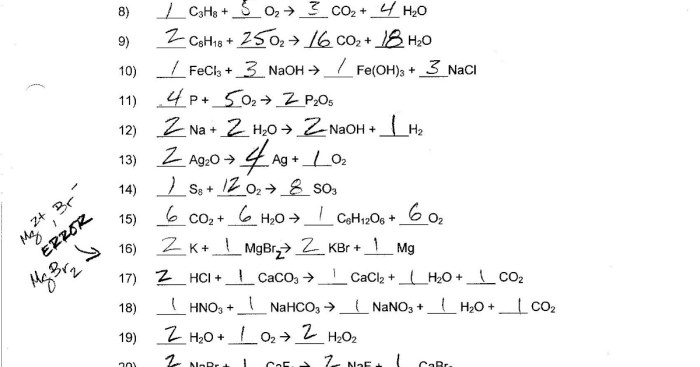
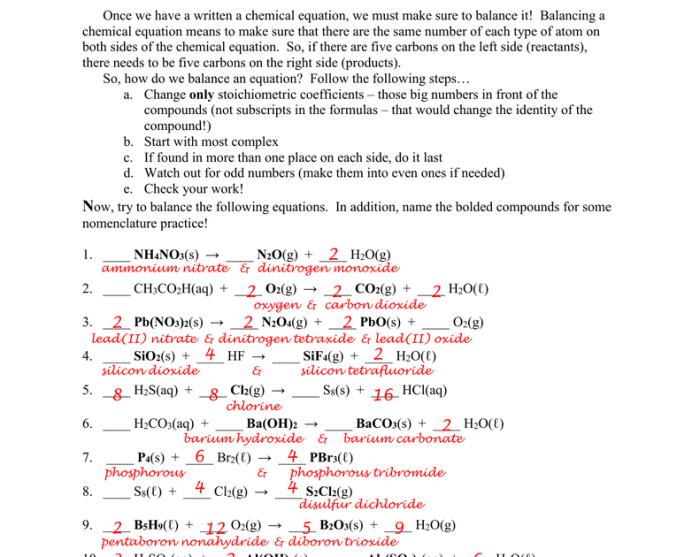
Balancing equations is the process of making sure that the number of atoms of each element is the same on both sides of a chemical equation. This is important because chemical reactions must follow the law of conservation of mass, which states that matter cannot be created or destroyed.
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. It is used to balance equations and to predict the amounts of reactants and products that will be involved in a reaction.
Balancing Equations Gizmo
The Balancing Equations Gizmo is a simulation that can be used to balance equations. The Gizmo provides a visual representation of the atoms and molecules involved in a reaction, and it allows users to experiment with different ways to balance the equation.
Methods for Balancing Equations
There are several different methods that can be used to balance equations. One common method is to use the half-reaction method. This method involves splitting the equation into two half-reactions, one for the oxidation of the reactants and one for the reduction of the products.
The half-reactions are then balanced separately, and the two balanced half-reactions are combined to form the balanced equation.Another common method for balancing equations is to use the oxidation number method. This method involves assigning oxidation numbers to each atom in the equation.
The oxidation numbers are then used to determine the number of electrons that are transferred in the reaction. The equation is then balanced by adding electrons to the appropriate side of the equation.
Examples of Balanced Equations, Balancing equations gizmo answer key pdf
The following are examples of balanced equations:* 2H 2+ O 2→ 2H 2O
- CH 4+ 2O 2→ CO 2+ 2H 2O
- Fe + 2HCl → FeCl 2+ H 2
Tips for Balancing Equations
Here are some tips for balancing equations:* Start by balancing the elements that appear in only one molecule on each side of the equation.
- Balance the elements that appear in more than one molecule by multiplying the coefficients of the molecules that contain those elements.
- Check your work by making sure that the number of atoms of each element is the same on both sides of the equation.
Applications of Balancing Equations
Balancing equations is an essential skill for chemists. It is used to predict the amounts of reactants and products that will be involved in a reaction, and it is also used to calculate the energy changes that occur during a reaction.
Balancing equations is also used in a variety of other applications, such as environmental chemistry, food chemistry, and pharmaceutical chemistry.
Clarifying Questions
What is the purpose of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element on the reactants’ side of the equation equals the number of atoms of that element on the products’ side. This reflects the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
How can I use the Balancing Equations Gizmo to balance equations?
The Balancing Equations Gizmo provides an interactive simulation that allows users to manipulate coefficients and observe the resulting changes in the equation. By adjusting the coefficients until the number of atoms of each element is equal on both sides, learners can balance equations efficiently and effectively.
What are some tips for balancing equations?
Start by balancing the most complex molecule first. Balance elements one at a time, beginning with those that appear in the greatest number of molecules. Use the Gizmo’s “Check” button to verify your work and identify any errors.