Liquid pentanol C5H12O and gaseous oxygen engage in a captivating combustion reaction, unveiling intriguing physical, chemical, and safety considerations. This discourse delves into the molecular structure, properties, reactivity, production, and combustion of pentanol C5H12O, shedding light on its multifaceted nature.
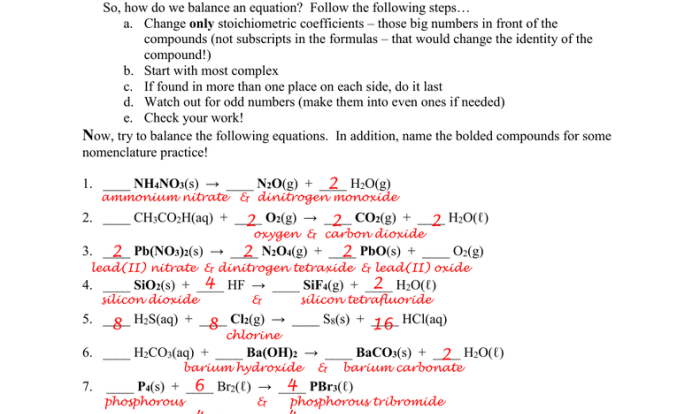
As pentanol C5H12O interacts with oxygen, a cascade of reactions unfolds, releasing energy and forming novel compounds. Understanding the intricate interplay between these substances is crucial for safe handling and harnessing their potential applications.
Physical Properties of Liquid Pentanol C5H12O
Pentanol C5H12O is a primary alcohol with a molecular structure consisting of a five-carbon chain with a hydroxyl group (-OH) attached to the primary carbon. It is a colorless liquid with a characteristic odor.
| Property | Value |
|---|---|
| Molecular weight | 88.15 g/mol |
| Boiling point | 138.4 °C |
| Melting point | -79.0 °C |
| Density | 0.819 g/mL |
| Refractive index | 1.410 |
Pentanol C5H12O is a polar molecule due to the presence of the hydroxyl group. It is soluble in water and other polar solvents, but it is also soluble in nonpolar solvents such as diethyl ether.
Chemical Properties of Liquid Pentanol C5H12O
Pentanol C5H12O is a reactive compound that can undergo a variety of chemical reactions. It reacts with acids to form esters, with bases to form salts, and with oxidizing agents to form aldehydes or ketones.One of the most common reactions of pentanol C5H12O is esterification.
In this reaction, pentanol C5H12O reacts with an acid to form an ester and water. For example, pentanol C5H12O reacts with acetic acid to form pentyl acetate and water:C5H12O + CH3COOH → CH3COOC5H11 + H2OPentanol C5H12O can also be oxidized to form aldehydes or ketones.
For example, pentanol C5H12O can be oxidized by potassium permanganate to form pentanal:
C5H12O + 4KMnO4 → 5C5H10O + 2H2O + 4MnO2 + KOH
Pentanol C5H12O is a good solvent for a variety of organic compounds. It is often used as a solvent for paints, varnishes, and other coatings.
Production and Uses of Liquid Pentanol C5H12O: Liquid Pentanol C5h12o And Gaseous Oxygen
Pentanol C5H12O can be produced by a variety of methods, including:* Hydrolysis of pentyl chloride
- Reduction of pentanal
- Fermentation of glucose
Pentanol C5H12O is used in a variety of industrial applications, including:* As a solvent for paints, varnishes, and other coatings
- As a cleaning agent
- As a fuel additive
- As a flavoring agent
The production and use of pentanol C5H12O is associated with a number of environmental and safety considerations. Pentanol C5H12O is a flammable liquid that can cause skin irritation and respiratory problems. It is also harmful to aquatic life.
Combustion of Gaseous Oxygen with Liquid Pentanol C5H12O
The combustion of pentanol C5H12O with gaseous oxygen is a highly exothermic reaction that produces carbon dioxide, water, and heat. The stoichiometry of the combustion reaction is as follows:C5H12O + 8O2 → 5CO2 + 6H2OThe enthalpy change for the combustion reaction is
3276 kJ/mol.
The products of the combustion reaction are carbon dioxide, water, and heat. Carbon dioxide is a greenhouse gas that contributes to climate change. Water is a vital resource for all life on Earth. Heat can be used to generate electricity or to provide warmth.
Safety Considerations for Liquid Pentanol C5H12O and Gaseous Oxygen

Pentanol C5H12O is a flammable liquid that can cause skin irritation and respiratory problems. It is also harmful to aquatic life. Gaseous oxygen is a non-flammable gas that supports combustion. It is important to handle and store pentanol C5H12O and gaseous oxygen safely.Pentanol
C5H12O should be stored in a cool, dry place away from sources of heat and ignition. It should be kept in a tightly closed container to prevent evaporation. Gaseous oxygen should be stored in a pressurized cylinder. It should be kept away from sources of heat and ignition.In
case of an accident involving pentanol C5H12O or gaseous oxygen, it is important to evacuate the area and call for help. If pentanol C5H12O comes into contact with skin, it should be washed off immediately with soap and water. If pentanol C5H12O is inhaled, the person should be moved to fresh air.
If gaseous oxygen is inhaled, the person should be given artificial respiration.
Essential Questionnaire
What is the molecular structure of pentanol C5H12O?
Pentanol C5H12O possesses a linear molecular structure, consisting of a five-carbon chain with a hydroxyl group (-OH) attached to the primary carbon.
What are the major industrial uses of pentanol C5H12O?
Pentanol C5H12O finds extensive use in the production of solvents, coatings, and fragrances, as well as an intermediate in the synthesis of other chemicals.
What safety precautions should be taken when handling pentanol C5H12O?
Pentanol C5H12O is flammable and can cause skin and eye irritation. Proper ventilation, protective clothing, and adherence to safety protocols are essential for safe handling.