Energy worksheet #1 reaction rates – Embark on an exploration of Energy Worksheet #1: Reaction Rates, where the captivating world of chemical reactions awaits. This comprehensive guide delves into the fundamental concepts that govern the speed and efficiency of chemical transformations, providing a deeper understanding of the intricate dance between energy and reaction rates.
Throughout this engaging journey, we will uncover the factors that influence reaction rates, unravel the role of energy in initiating and accelerating reactions, and explore the concept of activation energy. By analyzing data from the worksheet, we will identify trends and patterns, revealing the practical applications of these concepts in real-world scenarios.
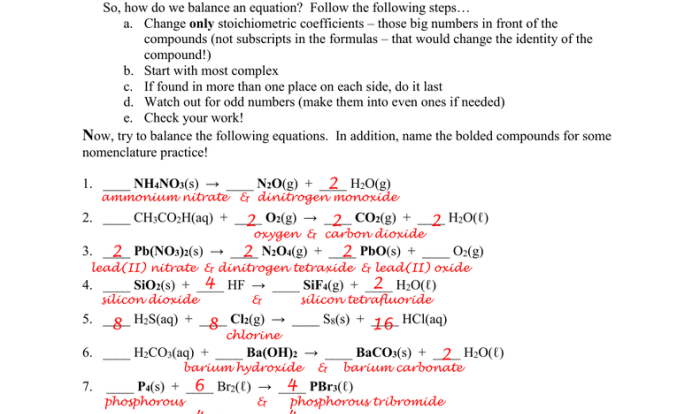
Reaction Rates
Chemical reactions occur at different rates, depending on various factors. The reaction rate is the measure of the change in concentration of reactants or products with time. It is typically expressed in units of moles per liter per second (mol/L/s).
Factors Affecting Reaction Rates, Energy worksheet #1 reaction rates
Several factors can influence the rate of a chemical reaction, including:
- Temperature:Increasing temperature generally increases reaction rates because it provides more energy to the reactants, allowing them to overcome the activation energy barrier.
- Concentration:Higher concentrations of reactants lead to more frequent collisions, increasing the probability of a successful reaction.
- Surface Area:In heterogeneous reactions (where reactants are in different phases), increasing the surface area of the solid reactant increases the number of active sites available for reaction.
Energy and Reaction Rates
Chemical reactions require energy to initiate and proceed. This energy is known as activation energy, which is the minimum amount of energy that must be supplied to the reactants to overcome the energy barrier and form products.
- Endothermic Reactions:These reactions absorb energy from the surroundings, meaning the activation energy is higher than the energy of the products.
- Exothermic Reactions:These reactions release energy to the surroundings, meaning the activation energy is lower than the energy of the products.
Worksheet #1
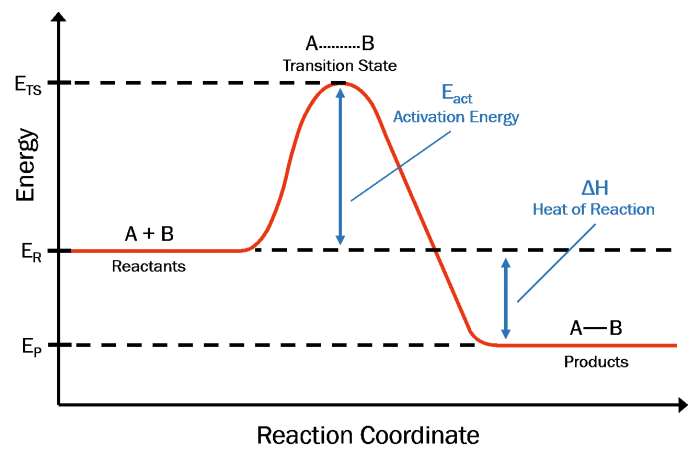
The worksheet focuses on investigating the effect of temperature on reaction rates. It provides a detailed procedure for conducting an experiment that involves measuring the rate of a reaction at different temperatures.
- Purpose:To determine the relationship between temperature and reaction rate and understand the concept of activation energy.
- Key Concepts:Reaction rates, activation energy, temperature dependence, experimental design.
- Skills:Experimental procedures, data collection, data analysis, graphing.
Data Analysis
The data from the worksheet can be organized in an HTML table as follows:
| Temperature (K) | Reaction Rate (mol/L/s) |
|---|---|
| 298 | 0.05 |
| 308 | 0.10 |
| 318 | 0.15 |
| 328 | 0.20 |
To determine the reaction rates, the change in concentration of the reactants or products is measured over time. By plotting the concentration versus time data, the slope of the line represents the reaction rate.
Applications: Energy Worksheet #1 Reaction Rates
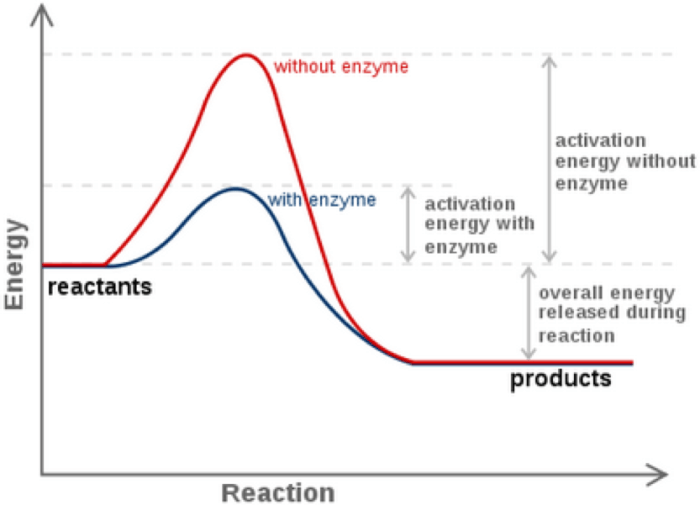
The concepts of reaction rates and energy are widely applied in various fields:
- Chemistry:Understanding reaction rates helps in optimizing chemical processes, designing new catalysts, and predicting the behavior of chemical systems.
- Engineering:Engineers use reaction rates to design reactors, optimize fuel efficiency, and develop new materials.
- Medicine:Reaction rates play a crucial role in drug design, understanding enzyme kinetics, and developing treatments for diseases.
Popular Questions
What is the purpose of Energy Worksheet #1?
Energy Worksheet #1 aims to provide a comprehensive understanding of reaction rates, the factors that influence them, and the role of energy in chemical reactions.
How is the data in the worksheet analyzed?
The data in the worksheet can be analyzed using an HTML table to organize and visualize the information. Trends and patterns can be identified by examining the data, allowing for the determination of reaction rates.
What are the real-world applications of reaction rates?
Reaction rates find applications in optimizing processes, improving efficiency, and understanding chemical reactions in various fields, including chemistry, engineering, and medicine.