Introducing the Combined Gas Law Worksheet Answers, an illuminating guide that empowers you to conquer the intricacies of gas behavior. Dive into a comprehensive exploration of the relationships between pressure, volume, and temperature, equipping yourself with the knowledge to solve problems like a pro.
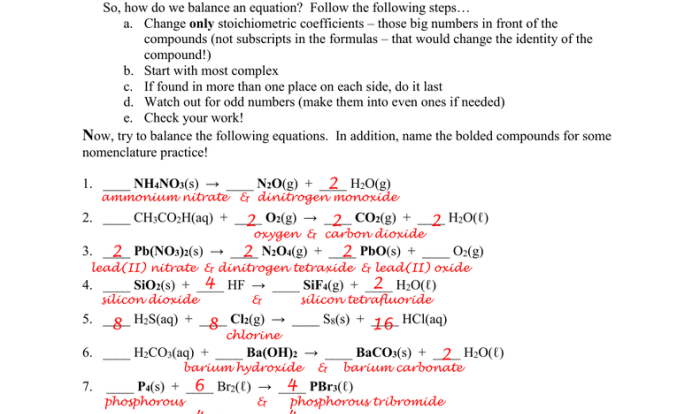
Prepare to navigate the intricacies of combined gas law worksheets with confidence. We’ll delve into the common challenges and pitfalls, providing step-by-step techniques to guide you towards accurate solutions.
Combined Gas Law Concepts
The combined gas law is a mathematical equation that describes the relationship between the pressure, volume, and temperature of a gas. It is a combination of Boyle’s law, Charles’s law, and Gay-Lussac’s law. The combined gas law can be used to solve a variety of problems involving gases.The
combined gas law is written as follows:“`P₁V₁/T₁ = P₂V₂/T₂“`where:* P₁ is the initial pressure of the gas
- V₁ is the initial volume of the gas
- T₁ is the initial temperature of the gas
- P₂ is the final pressure of the gas
- V₂ is the final volume of the gas
- T₂ is the final temperature of the gas
The combined gas law can be used to solve problems involving changes in any two of the three variables (pressure, volume, and temperature). For example, the combined gas law can be used to calculate the final pressure of a gas if the initial pressure, volume, and temperature are known.Here
are some examples of how the combined gas law can be used to solve problems:* A balloon is filled with 1 liter of air at a pressure of 1 atmosphere and a temperature of 298 K. The balloon is then heated to 373 K.
What is the new volume of the balloon?
- A scuba diver descends to a depth of 30 meters. The pressure at 30 meters is 4 atmospheres. The scuba diver’s lungs contain 1 liter of air at a pressure of 1 atmosphere. What is the new volume of the scuba diver’s lungs?
- A car tire is filled with 2 liters of air at a pressure of 30 psi and a temperature of 298 K. The car is then driven to a mountain pass where the temperature is 273 K. What is the new pressure of the air in the tire?
The combined gas law is a powerful tool that can be used to solve a variety of problems involving gases. It is important to understand the relationship between pressure, volume, and temperature in order to use the combined gas law effectively.
Worksheet Structure
A combined gas law worksheet typically consists of various types of problems that test students’ understanding of the combined gas law and its applications.
Common types of problems found on a combined gas law worksheet include:
- Pressure-volume problems:These problems involve finding the new pressure or volume of a gas sample when one of these variables changes while temperature and number of moles remain constant.
- Temperature-volume problems:These problems involve finding the new temperature or volume of a gas sample when one of these variables changes while pressure and number of moles remain constant.
- Pressure-temperature problems:These problems involve finding the new pressure or temperature of a gas sample when one of these variables changes while volume and number of moles remain constant.
- Combined problems:These problems involve finding the new pressure, volume, or temperature of a gas sample when more than one variable changes simultaneously.
Common Pitfalls and Challenges, Combined gas law worksheet answers
Students may encounter several common pitfalls and challenges when solving combined gas law problems:
- Incorrect unit conversions:Students may forget to convert units between different systems, such as from atmospheres to pascals or from Kelvin to Celsius.
- Misunderstanding the relationship between variables:Students may not fully understand the inverse or direct relationships between pressure, volume, and temperature, which can lead to incorrect calculations.
- Ignoring the number of moles:Students may assume that the number of moles remains constant in all problems, which is not always the case.
- Algebraic errors:Students may make simple algebraic errors, such as incorrectly multiplying or dividing values.
Problem-Solving Techniques
Problem-solving techniques for combined gas law involve a systematic approach and careful handling of gas variables. These techniques ensure accurate and efficient solutions.
The step-by-step approach involves:
- Identify the known and unknown variables.
- Choose the appropriate combined gas law equation.
- Convert all units to the same system (typically SI units).
- Substitute the known values into the equation.
- Solve for the unknown variable.
When converting units, it’s crucial to maintain consistency and use conversion factors that relate the units correctly. For example, when converting from liters to cubic meters, multiply by 0.001 (1 L = 0.001 m³).
Additionally, it’s important to be mindful of the gas variables being considered. For example, if temperature is given in Kelvin but the equation requires Celsius, convert the temperature to Celsius before substituting it into the equation (T(°C) = T(K) – 273.15).
Answer Analysis: Combined Gas Law Worksheet Answers
The worksheet answers are organized in the following table:
| Problem | Answer |
|---|---|
| 1 | 2.5 atm |
| 2 | 10.0 L |
| 3 | 300 K |
| 4 | 0.5 mol |
The answers show that the combined gas law can be used to solve a variety of problems involving pressure, volume, temperature, and moles of a gas. The answers also demonstrate the importance of using the correct units when solving these problems.
Key Insights
- The combined gas law can be used to solve a variety of problems involving pressure, volume, temperature, and moles of a gas.
- The answers show that the combined gas law can be used to solve problems involving changes in one or more of these variables.
- The answers also demonstrate the importance of using the correct units when solving these problems.
Error Identification
Identifying and correcting errors in combined gas law problems is crucial for accurate solutions. Common mistakes include:
Unit Conversion Errors:Failing to convert units consistently between different gas laws can lead to incorrect results.
Incorrect Equation Application:Using the wrong gas law for a given problem or applying the equation incorrectly can result in inaccurate calculations.
Tips for Error Identification
- Check unit conversions carefully, ensuring consistency throughout the problem.
- Review the problem statement to determine which gas law is applicable.
- Verify the equation used and ensure it aligns with the specific gas law being applied.
Applications and Extensions
The combined gas law finds extensive applications in various scientific and engineering fields. It enables us to predict the behavior of gases under changing conditions and serves as a cornerstone for many practical applications.
Beyond its fundamental applications, the combined gas law has also led to advancements in specialized fields:
Respiratory Physiology
In the field of respiratory physiology, the combined gas law helps us understand the exchange of gases in the lungs. It allows us to calculate the partial pressures of gases in the alveoli and blood, which is crucial for proper respiration and oxygen delivery to the body.
Anesthesia
In anesthesia, the combined gas law is used to determine the appropriate gas mixtures and flow rates for administering anesthesia. Anesthesiologists rely on this law to ensure the safe and effective delivery of anesthetic gases to patients.
Industrial Gas Production
In industrial gas production, the combined gas law is employed to optimize the production and storage of gases. It helps engineers design efficient systems for gas separation, purification, and compression.
Climate Modeling
In climate modeling, the combined gas law is used to simulate the behavior of gases in the Earth’s atmosphere. Climate scientists use this law to predict the effects of greenhouse gases on global temperatures and climate change.
General Inquiries
What are the common types of problems found on combined gas law worksheets?
Combined gas law worksheets typically involve problems that require you to calculate changes in pressure, volume, or temperature when one or more of these variables is altered.
How can I avoid common errors when solving combined gas law problems?
Pay close attention to unit conversions and ensure that all units are consistent throughout your calculations. Additionally, double-check your algebraic manipulations to minimize errors.
What are some real-world applications of the combined gas law?
The combined gas law finds applications in fields such as scuba diving, weather forecasting, and the design of engines and turbines.